The Office of Research Integrity and Institutional Review Board recently developed and approved major updates to the University’s Policies, Procedures and Guidance on Research with Human Subjects. It had been over 10 years since the policy was last updated, and this latest version includes all applicable updates from the 2018 Common Rule. Please take a quick look through the new version, which can be found on the IRB’s website: https://www.une.edu/sites/default/files/2020-08/UNE%20IRB%20PP%20%282020%29%20Final.pdf.
Some of the highlights include:
- Many administrative changes to make the policy more user-friendly and current.
- Only electronic IRB protocol submissions will be accepted now, so there is no need to mail or drop off hard copies anymore.
- Citi training is now valid for four (4) years, twice as long as the previous policy.
- There are new, additional elements of informed consent for expedited and full board protocols.
- The exemption categories have been significantly broadened. Expect to see protocols that previously would require expedited review to now be eligible for exemption.
- Some exempt projects will need to go through a “limited IRB review” to ensure information privacy and security for participants.
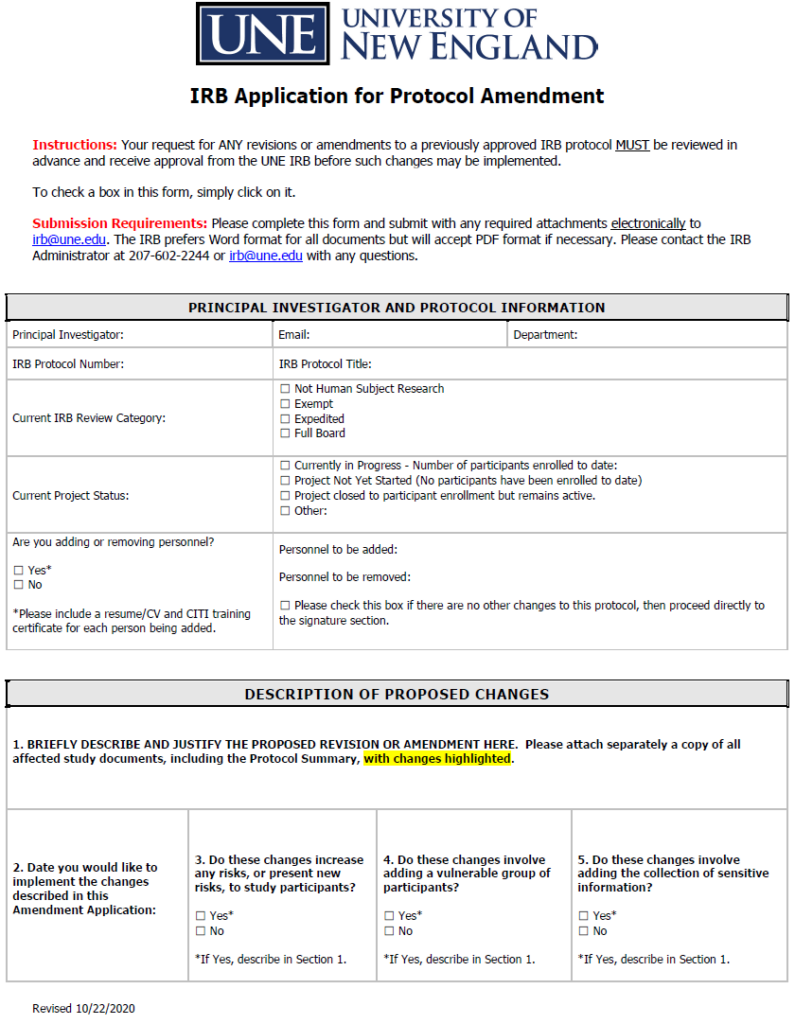
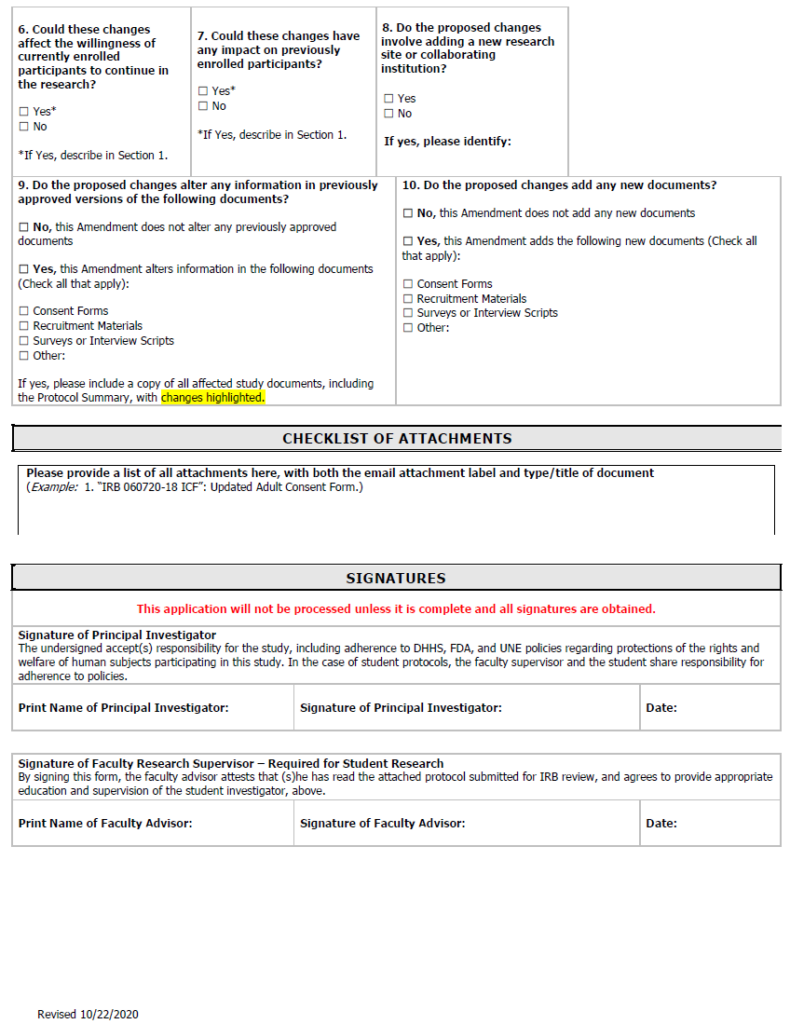
Updated IRB Amendment Application